This article is the first in a three-part series exploring the need and means to achieve improvement in aseptic processing of sterile biopharmaceutical products. Part 1 will present the current state and opportunity for improvement using innovative technology. Part 2 will further discuss some of the changes in strategy that might be needed for and result from the use and improvement of technology. Part 3 will present the impact of technology changes.
The manufacturing of sterile biopharmaceutical products using aseptic processing has not materially changed in decades, resulting in the same compliance-related issues and manufacturing challenges from year to year. While it is true that more and more companies are using isolators and barrier systems, the filling systems they encase are largely the same as conventional filling lines. The means to sterilize, transport, and fill products remains the same. Some companies are doing electronic batch records and data evaluation, but for the most part we still rely on varying levels of manual data entry with little connectivity of data gathering systems. PAT (process analytical technology), which showed promise in the early 2000s, has yet to take a foothold in aseptic processing. Operators still gown the same way. Control systems operate separately and independently, rarely exchanging information. Environmental monitoring relies on the placement, incubation, and reading of media. The result of this lack of technological advancement can be seen in:
- Lack of productivity: Today, many, if not most, companies operate largely on a four- to five-day per week, single-shift schedule, despite modern cleanrooms being designed to operate at high capacity levels around the clock.
- Lack of scientifically based decision making: Sterility assurance is based on the observation performance surrogates, such as media fills, environmental monitoring, and end-product testing, where the correlation between what is observed and the desired state of product sterility is vague.
- Underinformed decision making:We treat environmental microorganisms as direct indicators of product contamination, even though we have yet to quantify the scientific relationship between environmental conditions and product sterility.
- Human performance variation: We tend to rely on significant human intervention in filling operations, both conventional and isolator. Most material transfers and cleanroom disinfection are manual. Filling and environmental monitoring setup and performance are largely manual. We do little to consider ergonomics or process design for human error prevention, relying instead on often ineffective training methods to prevent and correct human performance issues.
- Fear to challenge: We acknowledge the need for improvement and the desire to embrace technology, but we place more emphasis on regulatory expectations than on critical and risk-based thinking, often fearing the risk of regulatory scrutiny and burden more than enjoying the benefits of innovative methods.
The future improvement of aseptic processing lies in better use of technology to meet the requirements of existing products and new therapies. Aseptic processing would benefit from automation, virtual reality, artificial intelligence, machine learning, and predictive modeling. These could lead to technology advances in contamination control using barrier systems, such as isolators and closed RABS (restricted access barrier systems), closed container filling, post aseptic lethal treatments, lower temperature terminal sterilization, rapid microbiological monitoring and testing methods. Continuous process manufacturing and parametric or real-time release for sterile products could bring higher productivity. Facilities of the future could be modular, with a trend to smaller, less complex, and easier to control critical product exposure spaces. All these technologies exist today and could benefit the industry.
However, despite huge advances in technology in other industries, aseptic processing of sterile medicinal products has struggled to adopt new technologies. There are many reasons for the reluctance of companies to embrace innovative aseptic processing technology, including regulatory questions and expectations, more burdensome qualification, additional time for pre-approval change notification, risk aversion, lack of knowledge about the technology, fear of the unknown, capital and operating costs, and lack of defined benefit. Whether these concerns are more perception than reality, they are all obstacles and barriers to innovation. Overcoming these obstacles has not been easy.
However, conditions in the industry are changing. This may be the right time for a technology revolution.
Regulators are ready.
Globally, health authorities and regulators are encouraging new approaches. EMA, PIC/S, and the FDA are suggesting and expecting the use of risk-based approaches to process design and control. This can be seen in the recent release of the EMA proposed revisions to Annex 1 (Manufacture of Sterile Medicinal Products), prepared by an international PIC/S working group that included representatives from Europe, the U.S. FDA, Australia, and Japan. The revision contains 43 specific recommendations for risk approaches, with many more implied. This opens the door for the industry to consider and propose new approaches.
The financial incentive is there.
Many of the blockbuster healthcare products are sterile injectable or implantable, produced by aseptic processing. This provides a strong business and financial driver for improving the productivity and efficiency of aseptic processes, and the rationale for modernizing aged facilities, including those used for manufacturing outsourcing.
The control strategies we have may no longer fit what we need.
The future will involve sterile ATMP (advanced therapy medicinal products), cell and gene therapy products, and personalized medicine. These new therapies require aseptic processing. Current aseptic processing approaches do not adequately address the unique needs of the manufacturing of autologous sterile products on the requisite small scale. New (and improved) science and risk-based approaches are needed. The tight release criteria and timeline for these products will provide the opportunity for rapid microbiological testing and in-process, real-time release of sterile products. These advancements will require and lead to changes in regulatory guidance, acceptance of new approaches, and use of innovative methods.
It is the right thing to do.
The effectiveness of biopharmaceutical products and therapies, the demand for these products from emerging growth markets, the cost of healthcare in the U.S., and product shortages have placed an emphasis on product availability and affordability. Life-changing and lifesaving therapies are only useful if they are available and affordable. This, coupled with shareholder pressure for better returns and performance, will add emphasis to the need for manufacturing process efficiency and productivity improvement.
Industry 4.0 is here and waiting.
There is a significant opportunity to acquire and link data at unprecedented levels. This information and knowledge can be used to better model, predict, and control aseptic processes. Virtual reality and simulation can be used to design facilities, plan and address problems in simulation without putting at risk actual plant operations, provide more effective training, more expeditious qualification, and higher levels of worker awareness. Artificial intelligence and machine learning can use data to optimize processes and process control.
The workforce is changing.
It is becoming more difficult to attract, train, and keep technical resources. The lack of enough skilled, qualified manufacturing personnel will result in the use of more automation, continuous process manufacturing, and smaller, less complex plants. In addition, innovation and cutting-edge technology will help attract the young technical talent needed by the industry.
The Opportunity For Aseptic Process Manufacturing Improvement
For the industry to meet the needs the future will demand, it must improve its use of technology. Critical thinking and science-based decision making will lead to better selection, adoption, and use of technology for aseptic process improvement. Critical thinking means making reasoned judgments that are logical and well thought out. It is a way of thinking in which one does not accept all arguments and conclusions but asks why and why not. To overcome the reluctance to use new technology, the industry must consider that:
- Traditional approaches to process control by monitoring and detection may be ineffective and should be reevaluated.
- Safe, tried-and-true traditional methods for process control may not represent the most effective way to provide process and product quality confidence.
- Requirements and activities with little or no value waste resources and reduce the ability to perform more important efforts, and therefore may pose a risk to public health.
- Risk-based thinking should be unbiased, scientifically sound, data-based, and avoid predetermined outcomes, and where that occurs, regulators should be open to alternate risk-based approaches.
Embracing New Technology Through A Partnership Of Manufacturers, Suppliers, And Regulators
The future of technology in the aseptic processing of sterile medicinal products will depend on and require three principle parties to work in a true partnership: sterile product manufacturers, health authority regulators, and technology suppliers. To be successful, all parties must recognize their respective roles and work in concert.
The role of sterile product manufacturers, as the users of technology, should be to identify manufacturing improvement needs. To do this effectively, sterile product manufactures must be willing to:
- Use critical, risk-based thinking to set useful design requirements and specifications.
- Not accept technology design risk, when better designs can mitigate that risk.
- Take chances and be willing to move out of their comfort zones, away from traditional approaches.
- Consider manufacturing reliability and technology to address areas where improvement is needed.
- Use the tools that may already be available, rather than wait for the “perfect solution.”
- Think ahead in plant and process designs and plan for future innovation.
- Share (nonproprietary) technology information and experience with others in the industry.
- Be open to health authority concerns, but resist looking to regulators for all the answers.
Once needs are identified, the role of technology suppliers is to provide technology to meet those needs. To do this effectively, the developers and suppliers of innovative technology must be willing to:
- Listen to user needs and regulator concerns.
- Be willing to modify existing products to meet user needs and address regulator concerns.
- Research innovative approaches that go beyond existing products.
- Strongly promote and communicate new ideas.
- Go beyond limitations, strive for perfection.
Once technology is offered, the role of regulators and health authorities is to judge whether the approach to using and controlling that technology is appropriate. To do this effectively, health authorities must be willing to:
- Share expectations, criteria for acceptance, and technology successes with the industry.
- Encourage and reward innovation through such means as PAC (post approval change) relief.
- Avoid discouraging new approaches and overburdening new technology efforts with unique and less-than-value-added requirements.
- Help navigate and facilitate innovative efforts through the regulatory process (e.g., the FDA’s ETT [Emerging Technology Team]).
- Be open to well thought out risk-based alternate approaches, when presented by industry.
Posted by: Dr. TarunChugh


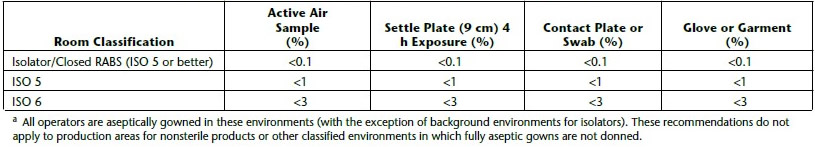
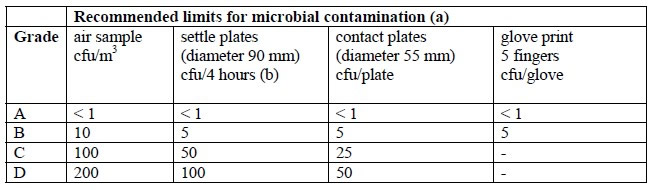



 a piece of equipment or process to provide absolute separation between the operator and product. The operator can perform tasks through half-suits or glove ports. Isolators provide a specific environment inside the isolator using HEPA filters. The environment can be positive pressure or negative, can have humidity control, oxygen control, use unidirectional airflow, and can either protect the product from the operator (as with aseptic processes) or protect the operator from the product (as with potent product handling).
a piece of equipment or process to provide absolute separation between the operator and product. The operator can perform tasks through half-suits or glove ports. Isolators provide a specific environment inside the isolator using HEPA filters. The environment can be positive pressure or negative, can have humidity control, oxygen control, use unidirectional airflow, and can either protect the product from the operator (as with aseptic processes) or protect the operator from the product (as with potent product handling). Non-viable monitoring wasn’t done in a sterility test isolator until a few years ago. Is it really necessary to know how many non-viable particles are in an isolator when the product is to be discarded? If passing sterility test results without knowing the non-viable counts in the sterility test isolator of 20 years was acceptable then, it certainly could be acceptable now.
Non-viable monitoring wasn’t done in a sterility test isolator until a few years ago. Is it really necessary to know how many non-viable particles are in an isolator when the product is to be discarded? If passing sterility test results without knowing the non-viable counts in the sterility test isolator of 20 years was acceptable then, it certainly could be acceptable now.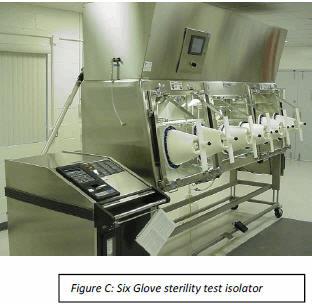 The hatchback window is closed and the decontamination cycle is initiated. Once the cycle is complete and the isolator is in run mode under positive pressure HEPA filtered air, testing can begin. At conclusion of testing, the hatchback window is opened and test samples are taken to the incubator and waste removed. The isolator is cleaned and is ready for the next sterility test. An isolator with no airlocks saves equipment cost and validation expenses.
The hatchback window is closed and the decontamination cycle is initiated. Once the cycle is complete and the isolator is in run mode under positive pressure HEPA filtered air, testing can begin. At conclusion of testing, the hatchback window is opened and test samples are taken to the incubator and waste removed. The isolator is cleaned and is ready for the next sterility test. An isolator with no airlocks saves equipment cost and validation expenses.


