Do Sterility Test Isolators Need To Be So Complicated?
In an article called “Paradise Lost” written by Jim Agalloco, president of Agalloco & Associates, Jim talks about the intent of early simple isolators for use in the pharmaceutical industry and how that expected “paradise” was never fully realized as isolator designs became more complex. He sums up his article by saying, “No regulator has mandated that isolators be designed to cleanroom standards, and the more we devoid ourselves of that misdirection the easier it will be to implement what should be the globally acknowledged superior technology of isolator”.
A good example of how simple isolators have been made complicated can be found in sterility test isolators. Isolators have been around the pharmaceutical industry since the early 1980s and in the nuclear industry (glovebox technology) since the 1950s. Isolators are used to create an airtight barrier or enclosure around  a piece of equipment or process to provide absolute separation between the operator and product. The operator can perform tasks through half-suits or glove ports. Isolators provide a specific environment inside the isolator using HEPA filters. The environment can be positive pressure or negative, can have humidity control, oxygen control, use unidirectional airflow, and can either protect the product from the operator (as with aseptic processes) or protect the operator from the product (as with potent product handling).
a piece of equipment or process to provide absolute separation between the operator and product. The operator can perform tasks through half-suits or glove ports. Isolators provide a specific environment inside the isolator using HEPA filters. The environment can be positive pressure or negative, can have humidity control, oxygen control, use unidirectional airflow, and can either protect the product from the operator (as with aseptic processes) or protect the operator from the product (as with potent product handling).
 a piece of equipment or process to provide absolute separation between the operator and product. The operator can perform tasks through half-suits or glove ports. Isolators provide a specific environment inside the isolator using HEPA filters. The environment can be positive pressure or negative, can have humidity control, oxygen control, use unidirectional airflow, and can either protect the product from the operator (as with aseptic processes) or protect the operator from the product (as with potent product handling).
a piece of equipment or process to provide absolute separation between the operator and product. The operator can perform tasks through half-suits or glove ports. Isolators provide a specific environment inside the isolator using HEPA filters. The environment can be positive pressure or negative, can have humidity control, oxygen control, use unidirectional airflow, and can either protect the product from the operator (as with aseptic processes) or protect the operator from the product (as with potent product handling).
The earliest uses of aseptic isolators were for sterility testing. Sterility test isolators make up most of the aseptic isolators in use and are available in many different sizes and configurations. Sterility test isolators do not need to be installed in a classified area. No formal requirement exists for a Grade D environment, but the area should be controlled to allow only trained personnel. The room should also have temperature and humidity control. The simple sterility test isolator shown in Figure A has a positive pressure blower, an inlet and outlet HEPA filter and operates under turbulent airflow. This unit also utilizes a PLC, which very early isolators did not have. However, since the introduction of hydrogen peroxide decontamination generators, automated communication between the isolator and generator are needed.
The US Pharmacopeia 1208 discusses the design of sterility test isolators. It states that the isolator must meet Class 100 conditions at rest but does not need to meet this classification during operations. Nor does the isolator need to meet an air velocity or air exchange rate criteria. Therefore, turbulent airflow is acceptable in a sterility test isolator; laminar airflow (unidirectional) is not required.
EU GMP Annex 1 focuses on the manufacture of sterile medicinal products where a Grade D background environment, at minimum, is required. Laminar airflow is needed inside the isolator for manufacturing. No mention is made of sterility test isolators.
Unidirectional airflow in sterility test isolators is not needed. It may help with distribution of decontamination vapors but this can be achieved with the proper number and placement of distribution fans inside the isolator. In some instances, half-suit isolators (See Figure B) are needed due to the size of product being tested. Unidirectional airflow would be disrupted by the half-suit.
Is particulate control needed in a destructive test? Should the test environment duplicate the filling environment? Certainly viable monitoring should be conducted in a sterility test isolator so that it can be shown there is no contamination inside the isolator during testing. This was particularly of interest in the past when a work station isolator, often equipped with a single or double half-suit due to its size, was meant to maintain “sterility” over weeks and sometimes months at a time.
A transfer isolator full of product and test supplies would be decontaminated daily and then connected to the work station isolator via a Rapid Transfer Port (RTP) as shown in Figure B. Materials would pass securely from the transfer isolator into the work station isolator without breaking the “containment” of either isolator. The work station isolator would be monitored daily for viable organisms using simple settle plates. The more sophisticated viable monitoring systems of today were not available. Yet the simple method was effective.
 Non-viable monitoring wasn’t done in a sterility test isolator until a few years ago. Is it really necessary to know how many non-viable particles are in an isolator when the product is to be discarded? If passing sterility test results without knowing the non-viable counts in the sterility test isolator of 20 years was acceptable then, it certainly could be acceptable now.
Non-viable monitoring wasn’t done in a sterility test isolator until a few years ago. Is it really necessary to know how many non-viable particles are in an isolator when the product is to be discarded? If passing sterility test results without knowing the non-viable counts in the sterility test isolator of 20 years was acceptable then, it certainly could be acceptable now.
Another “nice to have” on an isolator is an airlock. Airlocks are used to enter additional items or exit waste and finished media for incubation out of the isolator. Things to consider with airlocks are the size, number of glove ports needed and the environment. The environment of an airlock can be that of a neutral chamber with no pressure control and no airflow. It can also be positive pressure with turbulent airflow through HEPA filters or unidirectional airflow. Finally, the environment might need to be decontaminated with hydrogen peroxide or another agent. If many tests are being conducted in a day/week where the objective is to keep the main testing chamber “sterile” the airlock(s) can enter and exit materials by first adding the materials into the airlock, then decontaminating the airlock. Once the airlock decontamination cycle is complete (usually in much less time than a larger chamber) and the airlock reaches the appropriate positive pressure and airflow, the door to the main chamber is opened and materials transfer is performed.
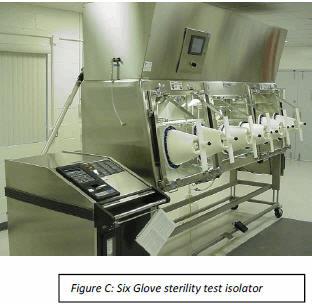
However, if testing is done once per day or a few times per week the airlock can be replaced with a good checklist and SOP. Here the main chamber hatchback window is opened and all test materials, product media, etc., is stored on the wire rack shelves in the isolator. Shelving size can easily be determined by simply placing all items needed for sterility testing on a lab bench and “taping” off the footprint of the shelves. This will help determine if a 4-glove isolator is sufficient or if a longer 6-glove isolator (Figure C) is needed. Once all items are in the isolator, the checklist is reviewed to ensure nothing has been forgotten.
 The hatchback window is closed and the decontamination cycle is initiated. Once the cycle is complete and the isolator is in run mode under positive pressure HEPA filtered air, testing can begin. At conclusion of testing, the hatchback window is opened and test samples are taken to the incubator and waste removed. The isolator is cleaned and is ready for the next sterility test. An isolator with no airlocks saves equipment cost and validation expenses.
The hatchback window is closed and the decontamination cycle is initiated. Once the cycle is complete and the isolator is in run mode under positive pressure HEPA filtered air, testing can begin. At conclusion of testing, the hatchback window is opened and test samples are taken to the incubator and waste removed. The isolator is cleaned and is ready for the next sterility test. An isolator with no airlocks saves equipment cost and validation expenses.
In conclusion, an effective sterility test isolator for low-volume testing can be a 4-glove isolator with a main chamber in 316L stainless steel with a safety glass hatchback window. The isolator will operate under positive pressure, with turbulent airflow through inlet and outlet HEPA filters. A PLC will control the isolator and also communicate with decontamination generators for an automatic decontamination. It can also include stainless steel wire rack shelving for supplies. For high-volume testing a similarly operated isolator with 6 gloves or even half-suits can be used. A transfer isolator can be employed as in the past to bring test materials to the work station isolator. The transfer isolator will also operate as a positive pressure, turbulent flow isolator. These simpler systems achieve the goal of eliminating false positives during testing and a lower cost.
By Gary Partington, Extract Technology
Posted By: Dr. Tarun Chugh



Thanks for sharing. A small addition, Station for viable monitoring during sterility test will help for monitoring and imvestigation tool.
ReplyDeleteYes, this is the need of today as CCP of Quality Assurance.
ReplyDeleteVery helpful
ReplyDelete